物理單位換算器
Unit Converter for Physics: Force, Velocity, Energy Made Simple
Physics problems require converting between dozens of unit systems—SI (metric), CGS (centimeter-gram-second), imperial, and specialized units like electronvolts. Whether you're solving mechanics problems, analyzing circuits, or studying thermodynamics, unit conversion errors cost you points.
This guide covers Newtons to pounds-force, m/s to mph, joules to electronvolts, and all essential physics conversions. You'll master SI units, avoid dimensional analysis mistakes, and convert any physics quantity confidently.
Need instant physics conversions? Our Unit Converter tool handles all scientific units with precision!

Understanding Physics Unit Systems
Physics uses three main systems, each with advantages for different applications.
SI Units (International System)
Base Units:
- Length: meter (m)
- Mass: kilogram (kg)
- Time: second (s)
- Electric current: ampere (A)
- Temperature: kelvin (K)
- Amount of substance: mole (mol)
- Luminous intensity: candela (cd)
Why SI Dominates:
- Coherent system: No hidden conversion factors in formulas
- Universal standard: Used worldwide in science and engineering
- Scalable: Prefixes (kilo, mega, nano) handle vast ranges
Example Coherence:
Force: 1 Newton = 1 kg·m/s² (exactly, no conversion factor)
Energy: 1 Joule = 1 kg·m²/s² = 1 N·m (exactly)
Power: 1 Watt = 1 J/s = 1 kg·m²/s³ (exactly)
{illustration: {
slug: "si-unit-system-hierarchy-diagram",
alt: "Pyramid diagram showing SI base units at bottom and derived units above with formulas connecting them",
caption: "SI system coherence: All derived units (Newton, Joule, Watt) connect to base units (kg, m, s) without conversion factors."
}}
CGS Units (Centimeter-Gram-Second)
Base Units:
- Length: centimeter (cm) = 0.01 m
- Mass: gram (g) = 0.001 kg
- Time: second (s)
Common CGS Derived Units:
- Force: dyne = 1 g·cm/s² = 10⁻⁵ N
- Energy: erg = 1 g·cm²/s² = 10⁻⁷ J
- Pressure: barye = 1 dyne/cm² = 0.1 Pa
Still Used In:
- Older physics textbooks
- Some electromagnetism formulas
- Astrophysics (occasionally)
Imperial/US Customary Units
Common Units:
- Length: foot (ft), inch (in), mile (mi)
- Mass: pound-mass (lbm), slug
- Force: pound-force (lbf)
- Energy: British Thermal Unit (BTU), foot-pound (ft·lb)
Engineering Note: US engineering often mixes systems (feet for length, pounds-force for force, BTU for energy), making conversions essential!
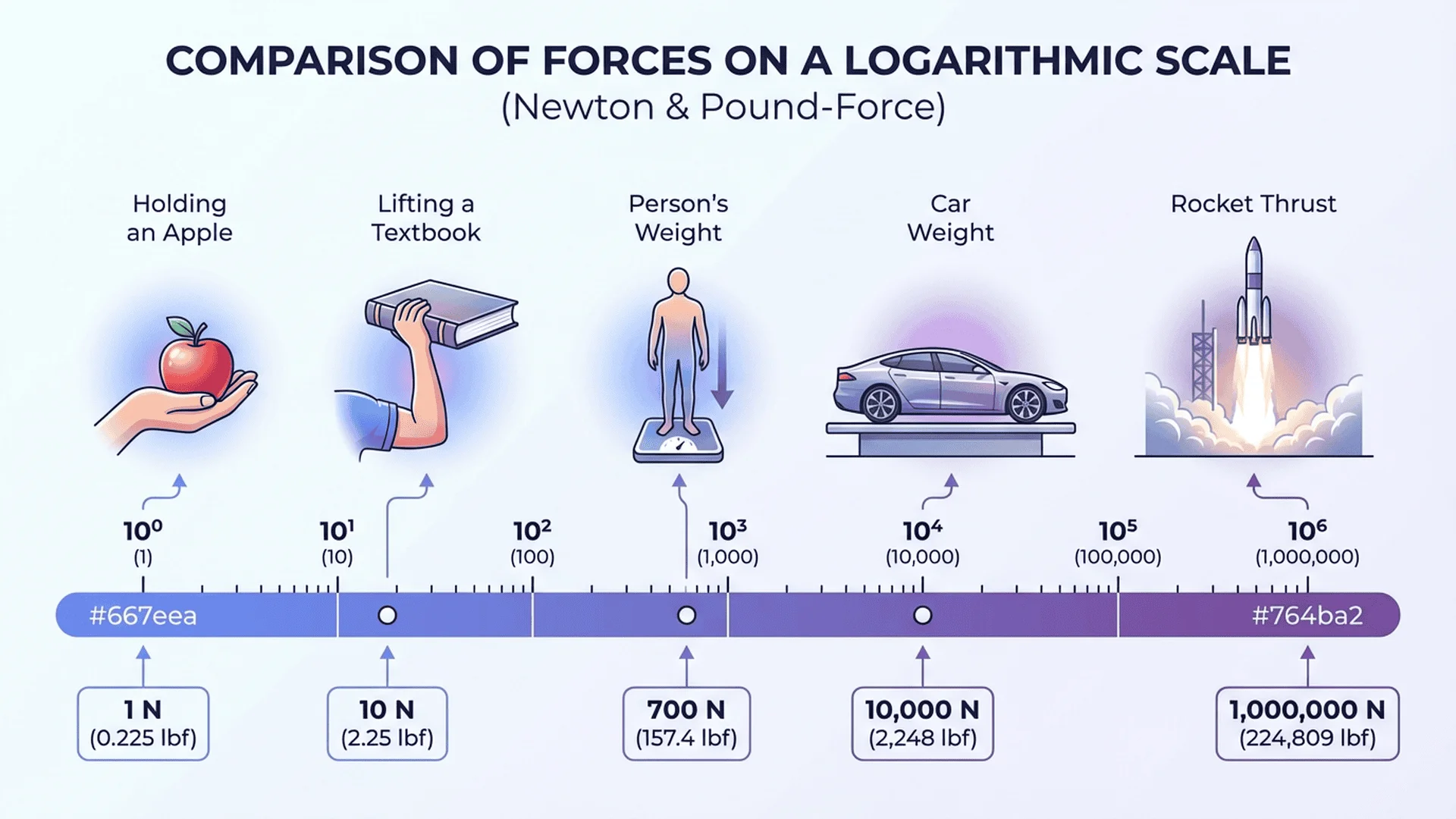
Force Conversions: Newtons, Pounds-Force, Dynes
Force measures push or pull—critical for mechanics and engineering.
Conversion Formulas
1 Newton (N) = 1 kg·m/s²
1 Newton = 0.224809 pounds-force (lbf)
1 Newton = 100,000 dynes (dyn)
1 pound-force = 4.44822 Newtons
1 dyne = 10⁻⁵ Newtons = 0.00001 N
Quick Conversion Table
| Force | Newtons (N) | Pounds-Force (lbf) | Dynes (dyn) |
|---|---|---|---|
| Tiny force | 0.001 N | 0.00022 lbf | 100 dyn |
| Apple falling | 1 N | 0.225 lbf | 100,000 dyn |
| Strong handshake | 100 N | 22.5 lbf | 10⁷ dyn |
| Car weight (1000 kg) | 9,810 N | 2,205 lbf | 9.81×10⁸ dyn |
| Rocket thrust | 1,000,000 N | 224,809 lbf | 10¹¹ dyn |
Real-World Examples:
- 1 N: Force to hold a small apple (100g mass)
- 10 N: Force to lift a 1 kg textbook
- 700 N: Average adult weight (70 kg × 9.81 m/s²)
- 4.45 N: 1 pound-force (1 lbf)
{illustration: {
slug: "force-units-comparison-scale",
alt: "Logarithmic scale showing force from holding apple (1 N) to rocket thrust (megaNewtons) with conversions to lbf",
caption: "Force spans 10+ orders of magnitude in everyday applications. Newtons are most convenient for human-scale physics."
}}
Common Force Calculation
Weight Formula:
Force (N) = Mass (kg) × Gravity (m/s²)
Weight = m × g
Example: 70 kg person on Earth
Weight = 70 kg × 9.81 m/s² = 686.7 N ≈ 154 lbf
Spring Force (Hooke's Law):
Force (N) = Spring Constant (N/m) × Displacement (m)
F = k × x
Example: Spring with k = 500 N/m compressed 0.1 m
F = 500 × 0.1 = 50 N ≈ 11.2 lbf
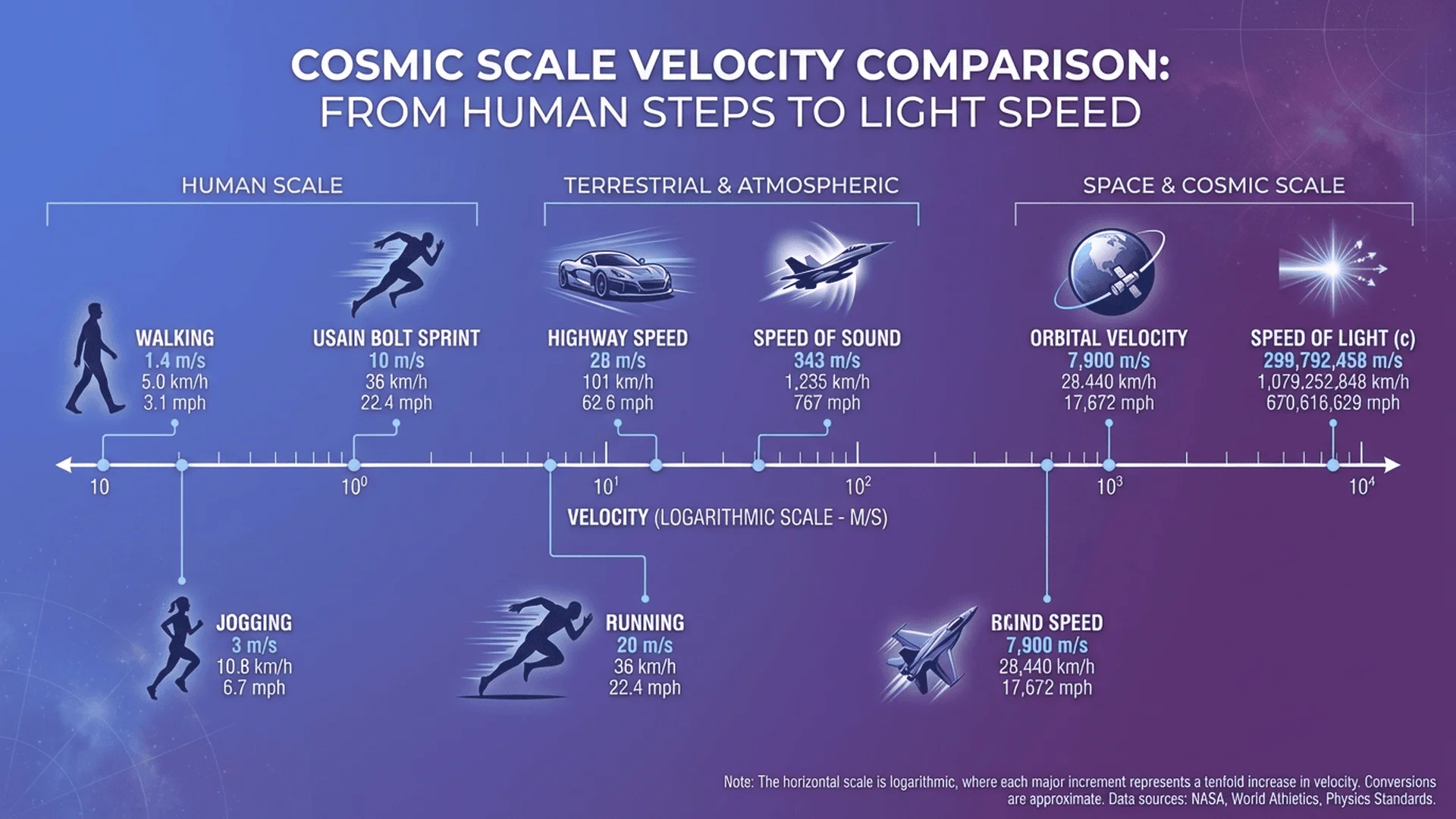
Velocity and Speed Conversions
Velocity measures distance per time—essential for kinematics.
Conversion Formulas
1 m/s = 3.6 km/h
1 m/s = 2.23694 mph (miles per hour)
1 km/h = 0.277778 m/s
1 mph = 0.44704 m/s
1 mph = 1.60934 km/h
Comprehensive Velocity Table
| m/s | km/h | mph | ft/s | Knots | Context |
|---|---|---|---|---|---|
| 0.5 | 1.8 | 1.1 | 1.6 | 1.0 | Slow walk |
| 1.4 | 5.0 | 3.1 | 4.6 | 2.7 | Average walk |
| 3.0 | 11 | 6.7 | 9.8 | 5.8 | Jogging |
| 10 | 36 | 22 | 33 | 19 | Usain Bolt sprint |
| 28 | 100 | 62 | 92 | 54 | Highway speed |
| 83 | 300 | 186 | 272 | 161 | High-speed train |
| 343 | 1,235 | 767 | 1,125 | 667 | Speed of sound (sea level) |
| 7,900 | 28,400 | 17,650 | 25,900 | 15,350 | Orbital velocity |
| 299,792,458 | 1.08×10⁹ | 6.71×10⁸ | 9.84×10⁸ | 5.83×10⁸ | Speed of light |
Physics Constants:
- Speed of sound (air, 20°C): 343 m/s = 1,235 km/h = 767 mph
- Escape velocity (Earth): 11,186 m/s = 40,270 km/h = 25,020 mph
- Speed of light (c): 299,792,458 m/s (exact, defined value)
{illustration: {
slug: "velocity-comparison-infographic",
alt: "Visual scale comparing velocities from walking (1 m/s) to speed of light with m/s, km/h, and mph labeled",
caption: "Velocity spans 9 orders of magnitude from human walking to light speed. Each unit system has its practical range."
}}
Acceleration Conversions
1 m/s² = 3.6 km/h/s
1 m/s² = 2.23694 mph/s
1 m/s² = 3.28084 ft/s²
Standard gravity (g) = 9.80665 m/s² (exact)
1g = 32.174 ft/s²
Common Accelerations:
- Gravity (g): 9.81 m/s² (Earth surface)
- Car acceleration: 2-4 m/s² (0.2-0.4g)
- Sports car: 8-10 m/s² (0.8-1.0g)
- Elevator: 1-2 m/s² (0.1-0.2g)
- Fighter jet: 50-90 m/s² (5-9g)

Energy Conversions for Physics
Energy appears in many forms: kinetic, potential, thermal, electrical. Here's how to convert between units.
Standard Energy Conversions
1 Joule (J) = 1 kg·m²/s² = 1 N·m
1 Joule = 0.239006 calories (small calorie)
1 Joule = 0.000239006 Calories (dietary Calorie)
1 Joule = 6.242×10¹⁸ electronvolts (eV)
1 Joule = 0.737562 foot-pounds (ft·lb)
1 Joule = 10⁷ ergs (CGS unit)
1 electronvolt = 1.602176634×10⁻¹⁹ Joules (exact)
Energy Scale Comparison
| Energy | Joules | eV | Calories | Context |
|---|---|---|---|---|
| Photon (visible light) | 3×10⁻¹⁹ J | 2 eV | 7×10⁻²⁰ cal | Red light photon |
| Thermal energy (300K) | 4×10⁻²¹ J | 0.026 eV | 10⁻²¹ cal | kT at room temp |
| ATP hydrolysis | 5×10⁻²⁰ J | 0.3 eV | 1.2×10⁻²⁰ cal | Cell energy currency |
| X-ray photon | 1.6×10⁻¹⁵ J | 10 keV | 3.8×10⁻¹⁶ cal | Medical imaging |
| Apple falling 1m | 1 J | 6.24×10¹⁸ eV | 0.239 cal | Mechanical energy |
| AA battery | 9,360 J | 5.84×10²² eV | 2,237 cal | 1.3V × 2Ah |
| Food Calorie | 4,184 J | 2.61×10²² eV | 1,000 cal | Dietary energy |
| Gallon of gas | 120 MJ | 7.49×10²⁶ eV | 28.7 Mcal | Chemical energy |
| TNT (1 kg) | 4.184 MJ | 2.61×10²⁵ eV | 1 Mcal | Explosive energy |
| Nuclear fission (¹U) | 3.2×10⁻¹¹ J | 200 MeV | 7.6×10⁻¹² cal | Single atom split |
Electronvolt (eV) Usage:
- Atomic physics: 1-100 eV
- Chemistry: 1-10 eV (bond energies)
- X-rays: 1-100 keV (kiloelectronvolts)
- Nuclear: 1-10 MeV (megaelectronvolts)
- Particle physics: 1-1,000 GeV (gigaelectronvolts)
{illustration: {
slug: "energy-scale-logarithmic-chart",
alt: "Logarithmic chart showing energy from single photon to nuclear bomb with joules and eV scales",
caption: "Energy spans 40+ orders of magnitude from quantum to macroscopic. Different units suit different scales."
}}
Learn more energy conversions in our Energy Conversion Guide.
Pressure Conversions
Pressure measures force per area—critical for fluid mechanics and thermodynamics.
Conversion Formulas
1 Pascal (Pa) = 1 N/m² = 1 kg/(m·s²)
1 Pascal = 0.000145038 psi (pounds per square inch)
1 bar = 100,000 Pa = 100 kPa
1 atmosphere (atm) = 101,325 Pa (exact)
1 atm = 14.6959 psi
1 atm = 1.01325 bar
1 psi = 6,894.76 Pa ≈ 6.895 kPa
1 torr = 133.322 Pa (1/760 atm)
1 mmHg = 133.322 Pa (same as torr)
Pressure Comparison Table
| Pressure | Pascals (Pa) | psi | atm | bar | mmHg | Context |
|---|---|---|---|---|---|---|
| Vacuum | 0 | 0 | 0 | 0 | 0 | Perfect vacuum |
| High altitude | 30,000 | 4.4 | 0.3 | 0.3 | 225 | Mount Everest summit |
| Atmospheric | 101,325 | 14.7 | 1.0 | 1.013 | 760 | Sea level |
| Tire pressure | 220,000 | 32 | 2.2 | 2.2 | 1,650 | Car tire |
| Scuba tank | 20,700,000 | 3,000 | 204 | 207 | 155,250 | Full diving tank |
| Deep ocean | 110,000,000 | 16,000 | 1,086 | 1,100 | 825,000 | Mariana Trench |
Special Units:
- Torr: Named after Torricelli, 1 torr = 1 mmHg ≈ 133.3 Pa
- Bar: Convenient for meteorology, 1 bar ≈ 1 atm
- Millibar (mbar): Weather maps use 1013 mbar = 1 atm
{illustration: {
slug: "pressure-units-comparison-diagram",
alt: "Diagram showing pressure units with atmosphere at center and conversions to Pa, psi, bar, and mmHg",
caption: "Atmospheric pressure (1 atm = 101.325 kPa = 14.7 psi = 1.013 bar) is the reference point for most pressure measurements."
}}
Learn detailed pressure conversions in our Pressure Conversion Guide.
Power Conversions
Power measures energy transfer rate—essential for electrical and mechanical systems.
Conversion Formulas
1 Watt (W) = 1 J/s = 1 kg·m²/s³
1 Watt = 0.00134102 horsepower (HP)
1 Watt = 3.41214 BTU/hr
1 horsepower = 745.7 Watts
1 horsepower = 550 ft·lb/s
1 BTU/hr = 0.293071 Watts
Power Examples
| Power Source | Watts | Horsepower | BTU/hr | Application |
|---|---|---|---|---|
| LED bulb | 10 W | 0.013 HP | 34 BTU/hr | Lighting |
| Human (resting) | 100 W | 0.13 HP | 341 BTU/hr | Metabolism |
| Athlete (peak) | 500 W | 0.67 HP | 1,706 BTU/hr | Cycling sprint |
| Electric motor | 3,730 W | 5 HP | 12,729 BTU/hr | Power tools |
| Car engine | 150,000 W | 201 HP | 511,821 BTU/hr | Sedan |
| Wind turbine | 2,000,000 W | 2,682 HP | 6.8M BTU/hr | 2 MW turbine |
| Power plant | 1,000,000,000 W | 1.3M HP | 3.4B BTU/hr | 1 GW plant |
Physics Problem Example:
A 70 kg person climbs 100m stairs in 2 minutes. What power?
Potential Energy = m × g × h = 70 × 9.81 × 100 = 68,670 J
Time = 2 minutes = 120 seconds
Power = Energy ÷ Time = 68,670 ÷ 120 = 572 W ≈ 0.77 HP
Learn more in our Power Conversion Guide.
Electrical Units
Electricity requires its own set of units and conversions.
Fundamental Electrical Units
Current (Amperes):
1 Ampere (A) = 1 Coulomb/second (C/s)
1 mA (milliamp) = 0.001 A
1 μA (microamp) = 0.000001 A
Voltage (Volts):
1 Volt (V) = 1 Joule/Coulomb (J/C)
1 Volt = 1 Watt/Ampere (W/A)
Resistance (Ohms):
1 Ohm (Ω) = 1 Volt/Ampere (V/A)
1 kΩ (kilohm) = 1,000 Ω
1 MΩ (megohm) = 1,000,000 Ω
Capacitance (Farads):
1 Farad (F) = 1 Coulomb/Volt (C/V)
1 μF (microfarad) = 10⁻⁶ F (most common)
1 pF (picofarad) = 10⁻¹² F
Charge (Coulombs):
1 Coulomb (C) = 1 Ampere × 1 second
1 electron charge (e) = 1.602176634×10⁻¹⁹ C (exact)
Electrical Power Calculations
Ohm's Law:
V = I × R
Voltage (V) = Current (A) × Resistance (Ω)
Example: 2 A through 100 Ω resistor
V = 2 × 100 = 200 V
Power Formulas:
P = V × I (Power = Voltage × Current)
P = I² × R (Power = Current² × Resistance)
P = V² ÷ R (Power = Voltage² ÷ Resistance)
Example: 120V circuit, 10A current
P = 120 × 10 = 1,200 W = 1.2 kW
{illustration: {
slug: "electrical-units-relationship-diagram",
alt: "Circular diagram showing relationships between voltage, current, resistance, and power with formulas",
caption: "Electrical units interconnect through fundamental laws. Know any two quantities and you can calculate the others."
}}
Temperature Conversions for Physics
Physics uses Kelvin (absolute temperature) instead of Celsius or Fahrenheit.
Conversion Formulas
Kelvin (K) = Celsius (°C) + 273.15
Celsius (°C) = Kelvin (K) - 273.15
Fahrenheit (°F) = (Celsius × 1.8) + 32
Rankine (°R) = Fahrenheit + 459.67 (absolute scale)
Important Temperature Points
| Event | Kelvin | Celsius | Fahrenheit | Rankine |
|---|---|---|---|---|
| Absolute zero | 0 K | -273.15°C | -459.67°F | 0°R |
| Liquid nitrogen | 77 K | -196°C | -321°F | 139°R |
| Dry ice sublimes | 195 K | -78°C | -109°F | 351°R |
| Water freezes | 273.15 K | 0°C | 32°F | 491.67°R |
| Room temperature | 293 K | 20°C | 68°F | 528°R |
| Body temperature | 310 K | 37°C | 98.6°F | 558°R |
| Water boils | 373.15 K | 100°C | 212°F | 671.67°R |
| Sun's surface | 5,778 K | 5,505°C | 9,941°F | 10,400°R |
Why Kelvin for Physics?
- Absolute scale: 0 K = no thermal energy (impossible to reach)
- No negative values: Simplifies thermodynamics math
- Direct energy relation: E = kT (Boltzmann constant × temperature)
Thermal Energy:
E_thermal = k × T
k = 1.380649×10⁻²³ J/K (Boltzmann constant)
At room temperature (293 K):
E = 1.38×10⁻²³ × 293 = 4.04×10⁻²¹ J ≈ 0.025 eV
Learn complete temperature conversions in our Temperature Conversion Guide.
Common Physics Conversion Mistakes
Mistake 1: Confusing Weight and Mass (60% of Errors!)
Wrong: "The astronaut weighs 70 kg on the Moon."
Right: "The astronaut has mass 70 kg (everywhere). Weight on Moon = 70 × 1.62 = 113 N."
Key Distinction:
- Mass (kg): Amount of matter (constant everywhere)
- Weight (N): Force = mass × local gravity (varies by location)
Mistake 2: Unit Dimension Errors
Wrong: Adding energy (Joules) to power (Watts)
Right: Energy = Power × Time (Joules = Watts × seconds)
Dimensional Analysis:
Check dimensions before calculating:
Force = mass × acceleration
[N] = [kg] × [m/s²] ✓ (dimensions match)
Energy ≠ Force + Velocity (dimensions incompatible!)
[J] ≠ [N] + [m/s] ✗ (nonsense!)
Mistake 3: Forgetting Squared/Cubed Factors
Wrong: "Area doubles → volume doubles"
Right: "Linear dimension doubles → area ×4, volume ×8"
Example:
Cube side = 2 m
Area = 6 × (2²) = 24 m²
Volume = 2³ = 8 m³
Double the side → 4 m
Area = 6 × (4²) = 96 m² (×4, not ×2!)
Volume = 4³ = 64 m³ (×8, not ×2!)
Mistake 4: Using Wrong Gravity Value
Problem: Using g = 10 m/s² when precision matters.
Accurate Values:
- Earth (sea level): g = 9.80665 m/s² (standard gravity)
- Earth (equator): g = 9.78 m/s²
- Earth (poles): g = 9.83 m/s²
- Moon: g = 1.62 m/s²
- Mars: g = 3.71 m/s²
When g = 10 is acceptable: Quick estimates, multiple-choice exams
When g = 9.81 required: Homework, labs, engineering calculations
{illustration: {
slug: "physics-conversion-mistakes-infographic",
alt: "Four-panel infographic showing common physics mistakes with wrong examples crossed out",
caption: "Avoid these four mistakes and your physics conversions will be correct 95% of the time."
}}
Dimensional Analysis: The Secret Weapon
Dimensional analysis ensures your conversions are correct by tracking units through calculations.
How It Works
Step 1: Write quantity with units
Step 2: Multiply by conversion factors (always = 1)
Step 3: Cancel units until you reach target
Example: Convert 60 mph to m/s
60 miles/hour × (1,609 meters/1 mile) × (1 hour/3,600 seconds)
= 60 × 1,609 ÷ 3,600 meters/second
= 26.8 m/s
Unit check: miles cancel, hours cancel → meters/second ✓
Example: Kinetic Energy Calculation
Car: mass = 1,500 kg, velocity = 30 m/s
Find kinetic energy in Joules
KE = ½ × m × v²
KE = 0.5 × 1,500 kg × (30 m/s)²
KE = 0.5 × 1,500 × 900 kg·m²/s²
KE = 675,000 J = 675 kJ
Dimension check: [kg·m²/s²] = [J] ✓
Pro Tip: Always write units in every step. If units don't match expected result, you made a mistake in the formula or conversion!
Using Our Unit Converter Tool
Solve physics problems faster with our Unit Converter.
Physics Features:
✓ All SI units (force, energy, power, pressure)
✓ CGS and imperial conversions
✓ Velocity, acceleration, and angular units
✓ Electrical units (V, A, Ω, F)
✓ Temperature (K, °C, °F)
✓ Instant dimensional analysis
✓ 100% free, works offline
{cta: {
title: "Convert Physics Units Instantly",
description: "Free scientific converter for all physics units. Perfect for homework, labs, and engineering calculations.",
primary_button: {
text: "Open Physics Converter",
url: "/tools/unit-converter",
priority: "⭐⭐⭐"
},
secondary_buttons: [
{
text: "View All Conversion Tools",
url: "/categories/conversion.html",
priority: "⭐⭐"
}
]
}}
Related Conversion Guides
Explore our complete conversion toolkit:
- Unit Converter Complete Guide - All conversion types in one place
- Energy Conversion Guide - Joules, eV, calories in detail
- Power Conversion Guide - Watts to horsepower formulas
- Pressure Conversion Guide - Pa, psi, atm conversions
- Temperature Conversion Guide - Kelvin to Celsius formulas
Need Technical Documentation? Check our Unit Converter Implementation guide for developers.
Conclusion: Master Physics Conversions
Physics conversions are essential for:
- Homework and exams: Converting between problem units and answer requirements
- Lab work: Ensuring consistent units across measurements
- Engineering: Matching specifications across different standards
- Research: Converting literature values to your working units
- Dimensional analysis: Verifying formula correctness
Key Takeaways:
1. SI units are coherent - no hidden conversion factors in formulas
2. Mass ≠ Weight - mass in kg, weight in Newtons (N)
3. 1 N = 0.225 lbf (force), 1 kg = 2.205 lb (mass)
4. 1 m/s = 3.6 km/h = 2.24 mph (velocity)
5. Always use dimensional analysis to verify conversions
Quick Mental Math:
- N → lbf: Divide by 4.5 (within 1%)
- m/s → mph: Multiply by 2.2 (within 2%)
- kPa → psi: Divide by 7 (within 1%)
- °C → K: Add 273 (exact enough for most work)
Best Practice: Write units in every calculation step. If final units don't match expected answer type, you made a mistake!
Use our Unit Converter tool for instant accurate conversions, or bookmark this guide for formulas and physics-specific examples.
Preparing for exams? Check our Unit Conversion Tables & Charts for comprehensive reference tables!
Frequently Asked Questions
Q: How do I convert Newtons to pounds-force?
A: Divide Newtons by 4.448 to get pounds-force (lbf). For example: 100 N ÷ 4.448 = 22.48 lbf. Newtons measure force in SI units, while pounds-force is used in US engineering.
Q: What's the difference between m/s and km/h?
A: m/s (meters per second) and km/h (kilometers per hour) both measure velocity. 1 m/s = 3.6 km/h. For example: 10 m/s = 36 km/h = 22.4 mph. m/s is used in physics; km/h for vehicles.
Q: How many joules are in 1 electronvolt?
A: 1 electronvolt (eV) = 1.602176634 × 10⁻¹⁹ joules (exact, 2019 SI redefinition). This tiny energy unit is essential for atomic and particle physics. 1 MeV = 1,602,176 J.
Q: Why does physics use SI units?
A: SI units (meter, kilogram, second, etc.) form a coherent system where derived units need no conversion factors. For example: 1 joule = 1 kg·m²/s² exactly, with no hidden multipliers. This simplifies calculations.
Q: What's the difference between weight and mass?
A: Mass (kg) is the amount of matter (constant everywhere). Weight (N) is the force due to gravity = mass × g (varies by location). A 70 kg astronaut weighs 686 N on Earth, but only 113 N on the Moon!
Q: How do I convert between CGS and SI units?
A: CGS uses cm-g-s, SI uses m-kg-s. Common conversions: 1 dyne = 10⁻⁵ N, 1 erg = 10⁻⁷ J, 1 barye = 0.1 Pa. Most modern physics uses SI exclusively.
Q: Why use Kelvin instead of Celsius?
A: Kelvin is absolute (0 K = no thermal energy), has no negative values, and simplifies thermodynamics math. Energy is directly proportional to Kelvin temperature (E = kT), which doesn't work with Celsius.
Q: How accurate should my conversions be?
A: Match precision to your measurement accuracy. Lab data to 3 sig figs? Convert to 3 sig figs. Exam estimate? 2 sig figs is fine. Engineering specs? Follow industry standards (often 4-5 sig figs).